Accuracy
Cognitive testing on a mobile device typically leverages touch detection, which uses projected-capacitive touch screens. Touch is detected through a cyclical “searching”, which presents concerns over consistency across devices and variable latency cycles depending on when the touch event occurred in the cycle. Sway’s patented motion measures provide more accurate measures that are consistent across iOS and Android.




Validation
All Sway cognitive tests undergo rigorous validation with high-speed cameras and third-party validation against gold standards. High speed cameras capture test accuracy to one millisecond, providing the highest level of accuracy available in mobile cognitive testing.
Reliability
Accuracy and consistency are both critical to the value of Sway‘s balance product. Reliability was shown in clinical studies analyzing test-retest variability in independent research studies of 30, 57, and 75 subjects. These results demonstrated strong test-retest reliability after an initial familiarization trial.


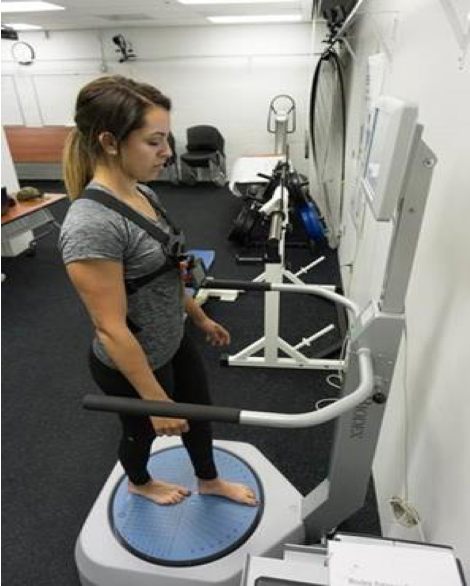

Force Platform Comparison
Sway has been repeatedly validated against force platform technology, the current gold standard for postural sway assessment. Sway was also better at differentiating between progressively more difficult balance conditions in third-party research studies.
BESS Comparison
The Sway Balance assessment has shown strong agreement (r > 0.83) with the Balance Error Scoring System (BESS) in laboratory settings, however the low inter-rater reliability associated with BESS makes it a challenge to implement. Sway is an effective sideline tool due to its objective metrics, simple user interface, and the time it takes to complete a test.

Medical Device - Sway Balance
Sway Balance is classified as a FDA cleared Class II medical device. Sway Balance is intended for use to assess sway as an indicator of balance. Individual suitability for assessment must be judged on a case by case basis, by a qualified individual including those certified and/or licensed in their state to prescribe and/or use balance devices such as certified athletic trainers and coaches, physical therapists, nurses, and physicians.
Sway Balance is a medical device and should only be administered with a full understanding of the testing procedure in a controlled environment on a flat, hard surface to ensure test repeatability. Sway Balance does not identify the presence or absence of clinical diagnoses, and is not intended as a stand-alone or adjunctive diagnostic device.
Sway Balance is contraindicated for balance assessment of hospitalized patients and individuals with compromised balance.
Medical Device - Sway Cognitive
Sway Cognitive is classified as a FDA Class II computerized cognitive assessment aid intended to provide an interpretation of the current level of cognitive function of an individual based on a battery of cognitive tasks. Sway Cognitive does not identify the presence or absence of clinical diagnoses and is not intended as a stand-alone or adjunctive diagnostic device. Sway Cognitive may only be used under the supervision of a practitioner licensed by law to direct its use.


